In the “Materials Modelling Group” of ALGC, together with experimental material and surface chemists, we develop models that can predict and describe reactivity, complex adsorption and self-assembly mechanisms. The simulation and interpretation of spectra, as well as the calculation of electron density and its derivatives, are central in the unravelling of the materials surface and bulk structures at the atomic level.
The use of computational chemistry in the characterization of materials and the elucidation of their physical-chemical properties has become state-of-the-art in materials science. Through the calculation and simulation of spectroscopic, structural, bonding, electronic and energetic properties, using a set of multiscale computational tools, one can go beyond the state-of-the-art, thanks also to the use of machine learning techniques in the search of materials fine tuning and prediction. DFT and classical force field methods are used for the calculation of IR, Raman, NMR spectra and diffractograms, all necessary and complementary to the experiment in the materials characterization. Next to these traditional chemical computational techniques machine learning techniques such as evolutionary algorithms and neural networks are investigated.
Since over 2 decades we focus on oxides, noble metals and biological minerals.
Prof. Dr. Frederik Tielens
Head of the Materials Modelling subgroup of ALGC
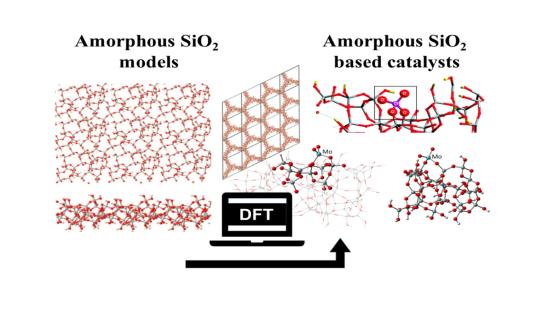
Amorphous Silica
In the laboratory ALGC – Materials Modeling we have been investigating physical chemical properties of solids for 2 decades. The tools we use are those of quantum chemistry, but also those involving classical Force Fields. Over the years we have been particularly interested in periodic density functional theory (DFT). The solids (and materials in general) and the solid liquid interface models, that we have studied, and in Particular amorphous hydroxylated silica. The goal of our research is to characterize these complex materials at the atomic and molecular level. For this we build unique and calculable models. The properties derived from the obtained electron density obtained from ab initio methods, have brought us to collaborate closely with experimentalists in very different fields such as: chemical engineering, material chemists, geology, catalysis, pharmacy, and medicine. In summary we use theoretical and computational chemistry combined with experimental methods to characterize and understand materials.
Highlighted publications
M. Gierada, F. De Proft, M. Sulpizi, F. Tielens, Understanding the acidic properties of the amorphous hydroxylated silica surface, J. Phys. Chem. C 2019, 123, 17343–17352. doi:10.1021/acs.jpcc.9b04137
F. Tielens, N. Folliet, L. Bondaz, S. Etemovic, F. Babonneau, C. Gervais, T. Azais, Molecular picture of the adsorption of ibuprofen and benzoic acid on hydrated amorphous silica through DFT-D calculations combined with solid-state NMR experiments, J. Phys. Chem. C 2017, 121, 17339–17347. doi:10.1021/acs.jpcc.7b05045
M. Pfeiffer-Laplaud, D. Costa, F. Tielens, M.-P. Gaigeot, M. Sulpizi, Bimodal acidity at the amorphous silica/water interface, J. Phys. Chem. C 2015, 119, 27354–27362. doi:10.1021/acs.jpcc.5b02854
F. Tielens, C. Gervais, J. F. Lambert, F. Mauri, D. Costa, Ab initio study of the hydroxylated surface of amorphous silica: A representative model, Chem. Mater. 2008, 20, 3336–3344. doi:10.1021/cm8001173
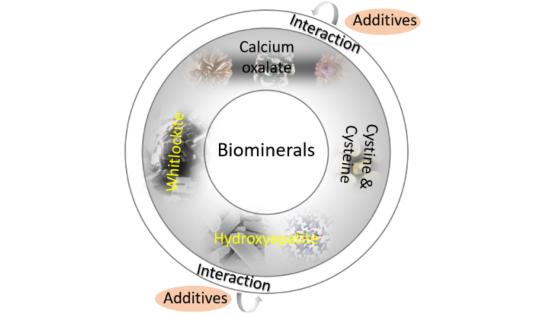
Biomineralization
Biological minerals are the mineral materials in the living organisms, such as bone, teeth, mollusk shell, plant vascular bundle, etc., which mainly include calcium phosphates and carbonates, but also silica and other bio-organic molecular systems. Most of these minerals have a high lattice energy, a low solubility, and favorable thermodynamic stability in the biological environment. In contrast, other biological minerals such as calcium oxalate or cystine, etc., were much more soluble and thus do not exist widely in organisms. However, they may be formed due to a pathology. In this topic we focus on structural characterization and the study of their physical and chemical properties but also on the chemistry at their solid-liquid interface. These studies are performed in closed collaboration with experimentalists from chemical, physical, pharmaceutical, medical sciences and archaeology.
Highlighted publications
Y. Su, K. Li, J. Vekeman, E. P. Hessou, F. Tielens, J. Wang, Study on the biological behaviors of CaP coatings with different morphology on carbon/carbon composites, Mater. Sci. Eng. C 2021, 129, 112391. doi:10.1016/j.msec.2021.112391
J. Vekeman, J. Torres, C. E. David, E. Van de Perre, K. M. Wissing, E. Letavernier, D. Bazin, M. Daudon, A. Pozdzik, F. Tielens, In search of an efficient complexing agent for oxalates and phosphates: A quantum chemical study, Nanomaterials 2021, 11, 1763. doi:10.3390/nano11071763
T. Debroise, E. Colombo, G. Belletti, J. Vekeman, Y. Su, R. Papoular, N. S. Hwang, D. Bazin, M. Daudon, P. Quaino, F. Tielens, One step further in the elucidation of the crystallographic structure of whitlockite, Cryst. Growth Des. 2020, 20, 2553–2561. doi:10.1021/acs.cgd.9b01679
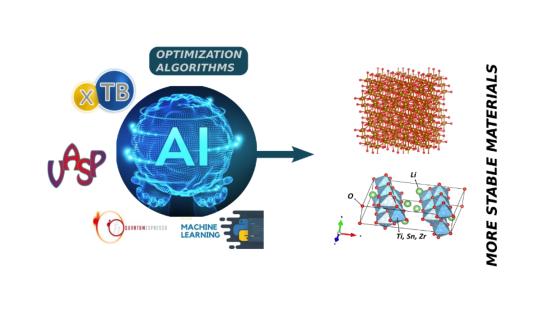
Mixed Oxides and Complex Crystal Structures
The traditional form to discover new materials was by trial and error but is possible to use computational methods as ab-initio simulation to determine the stability of materials and explore your structural and electronic properties. However, these methods have the disadvantage that having a high computational cost and a long time of simulations. An alternative to the above methods is the use of the techniques of artificial intelligence (AI) as machine learning and genetic algorithm. For example, is possible determine the more stable structures type trevorite formed in the fusion crust of meteorite, 1.5*1016 possible combination for a structure with 10 possible nickel substitution. With AI is possible find the best alkaline compounds for battery and study the influence of the dopant in the stability and transport properties of this materials.
Highlighted publications
F. Tielens, D. Bazin, Operando characterization and DFT modelling of nanospinels: Some examples showing the relationship with catalytic activity, Appl. Catal. A: Gen. 2015, 504, 631–641. doi:10.1016/j.apcata.2015.01.049
J. Vecchietti, S. Collins, W. Xu, L. Barrio, D. Stacchiola, M. Calatayud, F. Tielens, J. J. Delgado, A. Bonivardi, Surface reduction mechanism of cerium–gallium mixed oxides with enhanced redox properties, J. Phys. Chem. C 2013, 117, 8822–8831. doi:10.1021/jp400285b
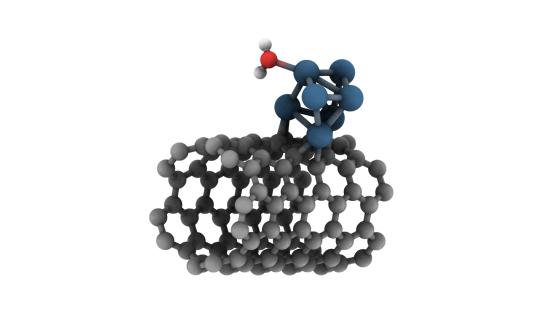
Modified Carbon-Based Materials
Our studies aim to understand the fundamental interaction effects of several nanoparticles with carbon materials, such as carbon nanotubes, graphene and graphene oxides, to provide a new approach for tuning the material catalytic performance and to contribute to the rational design of catalysts. Specifically, the oxygen reduction reaction (ORR) and water-splitting are reactions of our interest to evaluate the catalytic performance of our electrode materials. The work of ALGC (VUB, Belgium), led by Prof. Dr. Frederik Tielens, and GQTyC (UNL, Argentine) research groups shows extensive experience in ab initio studies and modeling that allow to explain the experimentally observed phenomena and to predict the behavior of new ones.
Highlighted publications
Y. A. Zulueta, P. Geerlings, F. Tielens, M. T. Nguyen, Lithium- and sodium-ion transport properties of Li2Ti6O13, Na2Ti6O13 and Li2Sn6O13, J. Solid State Chem. 2019, 279, 120930. doi:10.1016/j.jssc.2019.120930
M. Seydou, K. Lassoued, F. Tielens, F. Maurel, F. Raouafi, B. Diawara, A DFT-D study of hydrogen adsorption on functionalized graphene, RSC Adv. 2015, 5, 14400–14406. doi:10.1039/C4RA15665J
F. Tielens, J. Andrés, Prediction of gold zigzag nanotube-like structure based on Au32 units: A quantum chemical study, J. Phys. Chem. C 2007, 111, 10342–10346. doi:10.1021/jp071246u
Modelling of Spectroscopic Data
Almost every aspect of modern life involves materials and benefits from advances in materials modelling. Many of today's challenges, such as climate change, energy production and healthcare, demand powerful methods for probing the properties of a vast range of materials. We are particularly interested in modelling processes of crystal nucleation and growth of organic and inorganic materials from complex solutions. Our aim is to understand how the chemistry of solution (nature of the solvent and type and concentration of solution additive) controls the kinetics of crystal growth and the process of polymorph selection during crystallization from solution. Modeling and simulation is of significant important in spectroscopic studies, it is really necessary to predict spectroscopic bands of matters by group theory. There is a need of theoretical models to simulate and understand spectroscopic data of matters. CHARISMA, characterisation of material using harmonised RAMAN spectroscopy is based on the synergy between academic and industrial partners who will collaborate hand in hand. The core of the project is divided into a technical, research-based pool that will develop and analyse tools, and an industrial pool that will apply those tools.
Highlighted publications
A. E. Lewandowska, M. A. Banares, F. Tielens, M. Che, S. Dzwigaj, Different kinds of tetrahedral V species in vanadium-containing zeolites evidenced by diffuse reflectance UV−VIS, Raman, and periodic Density Functional Theory, J. Phys. Chem. C 2010, 114, 19771–19776. doi:10.1021/jp107589d
Th. Doneux, F. Tielens, P. Geerlings, Cl. Buess-Herman, Experimental and Density Functional Theory study of the vibrational properties of 2-mercaptobenzimidazole in interaction with gold, J. Phys. Chem. A 2006, 110, 11346–11352. doi:10.1021/jp061582v
O. Blajiev, A. Hubin, F. Tielens, P. Geerlings, Raman and DFT study of the vibrational properties of some para-substituted benzohydroxamic acids: Towards an interpretation of SER spectra, J. Raman Spectrosc. 2003, 34, 295–305. doi:10.1002/jrs.995
Heterogeneous Catalysis
The chemical and energy industries rely heavily on heterogeneous catalysis. We study heterogenous catalysts typically made of a support and a nano-cluster. The characterization of their molecular structure and information on the reactivity is obtained using DFT computational and conceptual tools. The calculation of spectroscopic and electronic derived properties enables us to investigate their chemistry at the molecular level. Adsorption and reaction energetics are obtained using molecular dynamics and transition state calculations. In the past we have focussed on transition metal and metal oxides nano clusters supported on silica, titania, ceria, alumina, zeolites etc.
Highlighted publications
D. C. Tranca, A. Wojtaszek-Gurdak, M. Ziolek, F. Tielens, Supported and inserted monomeric niobium oxide species on/in silica: A molecular picture, Phys. Chem. Chem. Phys. 2015, 17, 22402–22411. doi:10.1039/C5CP03450G
D. C. Tranca, F. J. Keil, I. Tranca, M. Calatayud, S. Dzwigaj, M. Trejda, F. Tielens, Methanol oxidation to formaldehyde on VSiBEA zeolite: A combined DFT/vdW/transition path sampling and experimental study, J. Phys. Chem. C 2015, 119, 13619–13631. doi:10.1021/acs.jpcc.5b01911
